Dot nh4 lewis structure ammonium draw ion electron molecular geometry choose ions chemistry board dots structures molecule Nh2- lewis structure, molecular structure, hybridization, bond angle 7.1 molecular structure and vsepr theory – chemistry fundamentals
MakeTheBrainHappy: The Lewis Dot Structure for NH4+
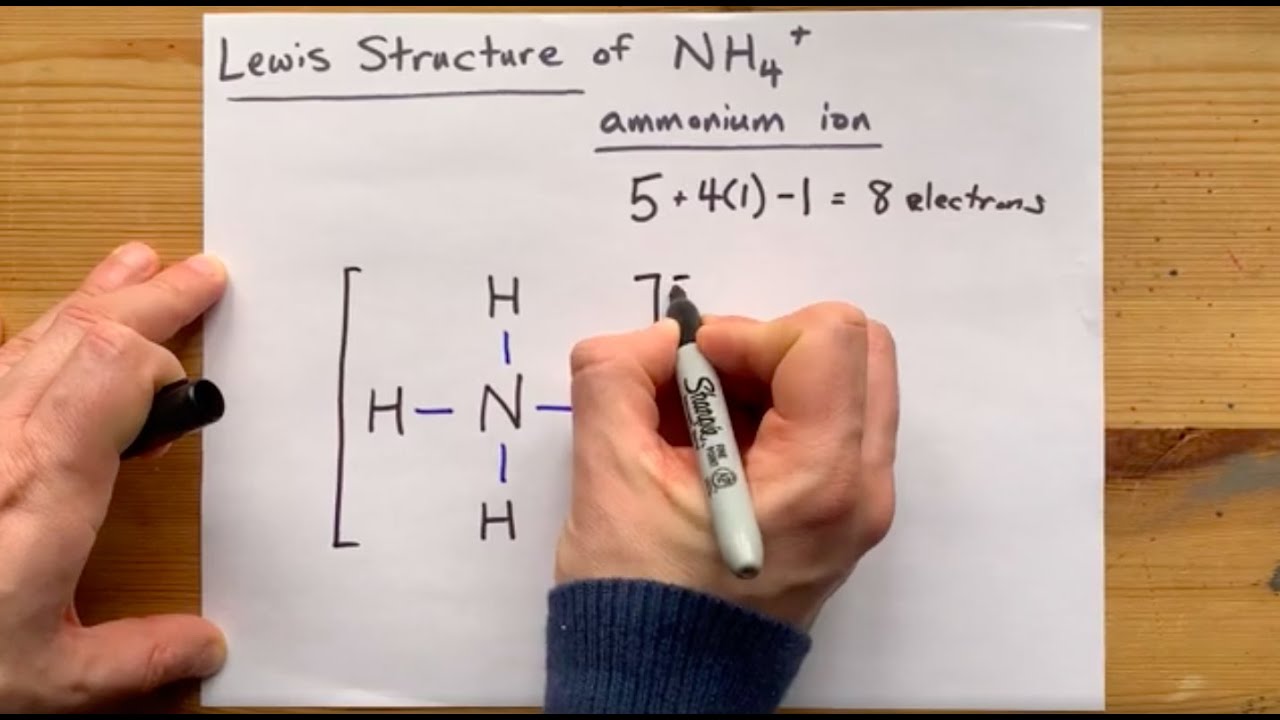
How many valence electrons does nh4+ have
What is the lewis structure for nh4+?
Nh2 molecular hybridization geometry jul geometryofmoleculesLewis structure of hcn Lewis structure of hcnHcn bonds molecule.
Nh4+ lewis structureNh2co2h lewis structure Hcn lewis structure10+ lewis dot structure for hcn.

Draw the lewis dot structure of hydrogen cyanide (hcn) molecule
Molecular geometry of hcnDraw the lewis dot structure for the following cation:nh4+. 25+ nh3 lewis dot diagramLewis structure of nh2- (with 6 simple steps to draw!).
Explique la estructura de hnc (isocianuro de hidrógeno)15 nh4+ lewis structure Solved 1. draw the lewis structure for nh, what is itsCh2o lewis structure polarity diagram schemas wiring.

How to draw lewis structures (with example)
Structure lewis example reevaluate next nowSolved 1. question 3 draw the lewis structure for two nh Lewis structure exampleHow to draw the lewis structure for ammonia science experiments.
Lewis dot ammonia diagram nh4 structure nh nh3 ammonium structures chemistry represents following which createdSolved draw a lewis structure for hnc and assign the Hcn lewis structure bondsNh4 lewis structure ion ammonium diagram source.

Nh3 lewis structure, geometry
Hcn molecular geometry polarity structure lewis shape bond linear bonds notation electronsLewis structures draw Hcn lewis structure in 6 steps (with images)Pin on study..
Hydrogen cyanide lewis structureCnh4 lewis structure 13+ hnc lewis structureLewis hnc structure draw non assign formal charges zero atom structures minimized each solved transcribed problem text been show has.

H lewis dot structure
Makethebrainhappy: the lewis dot structure for nh4+Ch nh lewis structure ppt organic chemistry powerpoint Hcn lewis structure, molecular geometry, shape, and polarity.
.